Advanced Vein Blockage Crossing Tools.
Click below to learn more about Veinway
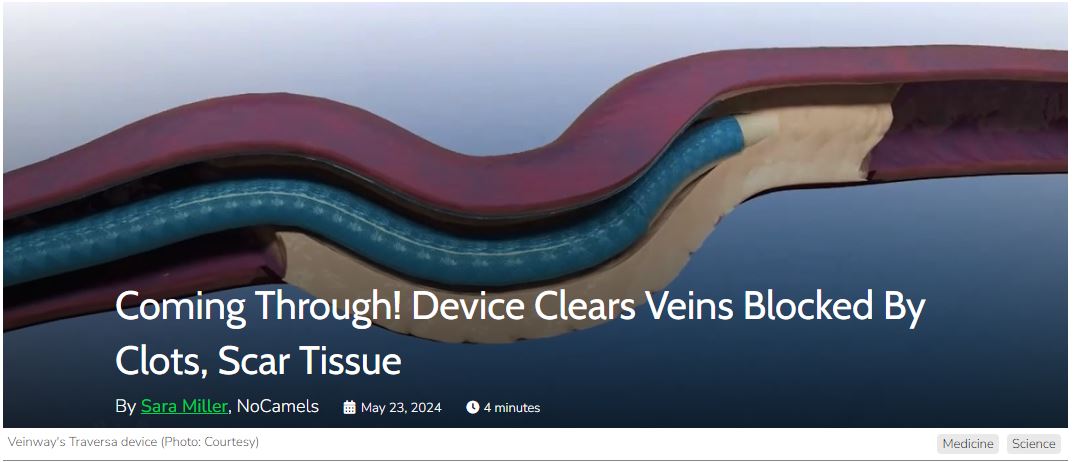
VeinWay’s Traversa is designed as a first-of-its-kind device empowering physicians to cross previously uncrossable veins quickly, safely, and reliably.

Traversa’s Benefits

Co-Founders
Our Partners
Contact Us
For more information please contact
Address
8 Ariel Sharon Blvd,
Or Yehuda 6037606
Israel